Equine infectious anemia (EIA) is geographically widespread throughout the world. Few countries can claim disease freedom, with the exception of Japan and Iceland. EIA continues to pose a threat to equine industries globally because of the potential risk of spread of the virus through international movement of horses. Of the equid species known to be susceptible to the virus, horses and ponies are more likely to develop severe clinical manifestations of the disease than are donkeys or mules, in which infection is most frequently subclinical. However, many horses also experience asymptomatic infection after initial exposure to the virus.
Etiology and Pathogenesis:
The causal agent of equine infectious anemia is an RNA virus, classified in the Lentivirus genus, family Retroviridae. The virus is readily inactivated by most common disinfectants, such as bleach, ethanol, iodophore disinfectants, phenolic compounds, glutaraldehyde, and formalin. Because bleach-based and ethanol disinfectants are readily inactivated in the presence of organic material, for example manure or soil, contaminated surfaces must first be cleaned thoroughly of such matter using soap and water before treating with a disinfectant. Pressure washing of a soiled surface is contraindicated because of the risk of aerosolization of potentially infectious blood or other body fluids on wall or floor surfaces.
In equids infected with EIA virus, there is a very close relationship between the development of overt signs of disease and the amount of virus present. Virus is found free in plasma or associated with monocytes and macrophages in infected animals. Virus burdens reach their highest levels during febrile episodes, after which they decline. It has been shown that the concentration of EIA virus in tissues must reach a threshold level to trigger a clinical response. The potential of a viral strain to induce disease is largely due to its replicative capacity or pathogenicity in the infected host.
The pathology of EIA-mediated disease is a consequence of macrophage infection that in turn interferes with host-cell gene expression; this leads to increased production of pro-inflammatory mediators or cytokines, in particular TNF-alpha, IL-1, IL-6, and transforming growth factor beta. Aside from the latter activating the arachidonic pathway that results in increased production of prostaglandin E2 and the induction of a febrile response, these cytokines may also cause thrombocytopenia. Increased production of TNF-alpha may also be partly responsible for the anemia that develops in EIA virus-infected equids by virtue of its ability to inhibit erythropoiesis.
Aside from the foregoing role that pro-inflammatory cytokines play in the pathogenesis of EIA, adaptive immune responses are also involved in the pathology of the disease. Platelets from infected horses have significant amounts of bound IgG or IgM, which result in their immune-mediated destruction, contributing to both splenomegaly and hepatomegaly.
There is reason to believe that cell-mediated and not humoral immune responses are responsible for initial control of EIA virus infection. Once acute viral infection has been controlled, the infected individual will remain free of overt signs of disease until a variant virus emerges that can evade the host’s immune system.
Epidemiology and Transmission of Equine infectious anemia:
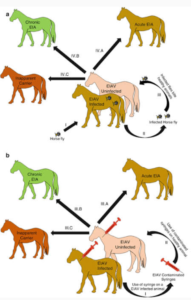
All equids infected with EIA virus remain lifelong carriers. Such individuals constitute the natural reservoir of the virus and ensure its perpetuation in equid populations over time. The combination of frequent carriers and mechanical transmission by blood-feeding insects explains why EIA is found in equine populations in a wide range of climatic zones and countries around the world.
Although EIA is usually considered a blood-borne infection, all body fluids and tissues should be regarded as potentially infectious, especially during febrile episodes when viral levels are high. Evidence of EIA virus has even been found in nasal swabs and in swabs taken from the buccal cavity and genitalia. There are limited data to suggest that infectious virus can be present in milk. EIA virus can also be passed to foals in utero. Evidence in support of venereal transmission is questionable; it is unlikely to occur unless semen is contaminated with blood in the case of an infected stallion.
There is circumstantial evidence suggesting that EIA virus may, under exceptional circumstances, also be transmitted via the respiratory route through aerosolization of blood when an infected horse is bleeding from the nostrils.
Transmission of EIA by biting flies is purely mechanical; the virus does not replicate in the insect. The chances of transmission of EIA among horses in close proximity to one another has been shown to be directly proportional to the volume of blood retained on the mouthparts of an insect after feeding. Based on this, horse flies, deer flies, and to a lesser extent, stable flies, are the most efficient vectors of the virus. It is also because the bites are irritating and trigger host defensive behavior that interrupts the flies’ feeding routine and results in their seeking out another susceptible host to complete their blood meal.
EIA transmission is influenced by the number and species of flies, density of the horse population, level of viremia in the host, and quantity of blood transferred. Infections are especially common in hot, humid countries of the world with very large biting fly populations. Symptomatic, febrile horses are more likely to transmit the disease than animals with inapparent infections.
Aside from the natural transmission of EIA by blood-feeding insects, the disease can also be readily transmitted iatrogenically through the re-use of blood-contaminated syringes and needles, surgical instruments, dental equipment, and IV sets and by the transfusion of infective blood or blood products. The virus is purported to persist for up to 96 hours on contaminated hypodermic needles. The importance of iatrogenic spread of EIA cannot be overstated. It has become increasingly common in some countries among a certain element of the equine industry that is indifferent to the inherent risks involved and the potential for dissemination of the virus.
Clinical Findings:
The clinical findings and course of infection with equine infectious anemia virus are variable, depending on the virulence of the virus strain, viral dose, and susceptibility of the horse. After an incubation period of 15–45 days or longer in naturally acquired cases of infection, classic cases of the disease have been described as progressing through three clinical phases. An initial or acute episode lasting 1–3 days is characterized by fever, depression, and thrombocytopenia. Because these signs can be mild and transitory, they are often overlooked or misdiagnosed. Typically, this initial phase is followed by a prolonged period associated with:
- recurring episodes of fever
- depression
- thrombocytopenia
- increased heart and respiration rates
- anemia
- jaundice
- petechiation on mucous membranes
- epistaxis
- dependent edema
- muscle weakness
- loss of condition
Read more about different diseases by visiting our website
Diagnosis of Equine infectious anemia:
Diagnosis is based on serologic tests
The clinical signs of acute equine infectious anemia are often nonspecific and not definitive of the disease. Accordingly, a provisional clinical diagnosis must be confirmed by demonstration of antibodies to the virus in blood. Laboratory confirmation of a suspect case of EIA needs to be pursued without delay. A wide range of infectious and noninfectious diseases can clinically resemble and be confused with EIA. These include:
- equine viral arteritis
- purpura hemorrhagica
- piroplasmosis
- leptospirosis
- severe strongyliasis or fascioliasis
- phenothiazine toxicity
- autoimmune hemolytic anemia
Although the internationally accepted serologic test is the agar gel immunodiffusion or Coggins test, there is increasing acceptance of a variety of ELISA tests, either competitive or synthetic antigen-based, because they can provide rapid results. Because ELISA tests can give a higher rate of false positives, all positive ELISA results must be confirmed by the Coggins test. When used in combination, ELISA and agar gel immunodiffusion tests provide the highest level of sensitivity combined with specificity. The Western blot is a supplemental test that can be resorted to in cases of conflicting results with other diagnostic tests.
A problem with available serologic tests is that they can give negative results when testing sera collected within the first 10–14 days of infection. Whereas the vast majority of horses infected with EIA virus will have seroconverted by 45 days, there have been exceptional cases in which the interval has been ≥90 days.
Virus detection assays such as reverse transcription PCR are not routinely used to diagnose EIA. Notwithstanding their sensitivity, they may not detect virus in carrier horses with very low viral loads. Although the animal inoculation test is highly sensitive for detection of EIA virus, for logistic and economic reasons, it is no longer in vogue as a means of diagnosis of EIA.
Treatment of Equine infectious anemia:
No treatment is available
There is no antiviral treatment or cure for equine infectious anemia. Because confirmed cases of the disease are lifelong carriers of the virus, they are usually euthanized. The alternative to euthanasia is permanent isolation and quarantine of the infected animal at a distance of at least 200 yards from all other equids on a premises. Advocating supportive care under such circumstances is irrelevant.
PREVENTION AND CONTROL:
Specific measures to prevent/control EIA can be summarized as follows:
- Infected horses become lifelong carriers and pose a risk of infection to other horses. Management options for an EIA-positive horse are euthanasia or lifetime quarantine, with permanent isolation at least 200 yards away from noninfected horses.
- Prevention is key to stopping the spread of EIA.
- Use a sterile needle, syringe, and IV set for all injections or treatments.
- Disinfect dental, tattoo, surgical equipment, lip chains, and bits thoroughly between horses. Remove all debris and blood with soap and water before disinfection.
- Only administer commercially licensed blood or blood products.
- Keep open wounds clean and covered, if possible.
- Use a sterile needle and syringe each time when puncturing a multidose medication bottle.
- Use sterile technique when drawing up and administering medications.
- Require proof of a recent negative EIA test upon introduction of a horse onto a premises for the first time.
- Practice good fly control by regular mucking out of stalls, proper disposal of manure away from horse stabling areas, and using fly sprays or natural predators to minimize fly presence.

Sir ! Kia ye zoonotic h ?
Very informative and clearly explained
Very informative and well explained😍
Very informative and well explained
Very informative sir 😍
Fantastic literature
Informative
Very interesting #
Great information for students and farmers . Thank you 😊 sir Allah bless you 🌹🙏
Very informative article for animals lover to read it.
Very informative article for animals lover to read it
Very informative notes sir g 🌹
Yes dear Jahan Haider this article is very informative especially for Horses 🐴🐎 lovers to get awareness regarding this problem. ❤️👍
Today topics Explain in easy word very important topics dear sir 😍😍
Very informative sir G